Focusing on the advantages of mRNA vaccine cutting-edge technology, seizing new opportunities in the industry
In late 2019, novel coronavirus pneumonia (coronavirus disease 2019, COVID-19) swept the globe with significant impact on the global economy as well as human health. Up to now, COVID-19 still spreads rapidly around the world. Vaccination is an important way to prevent and control the spread of this virus.
Back in 2021, the large-scale vaccination of mRNA in Europe and America sufficiently proved the excellent protective performance and safety of mRNA vaccines. Considering the continuation of COVID-19 and its outbreak in Shanghai recently, the sequential booster of vaccination becomes even more necessary. According to Dr. Zhong Nanshan, “We must enhance immunization. Heterovaccines should be used for sequential vaccination.” In the meanwhile, the 14th Five-Year Plan for the development of pharmaceutical industry pointed out that we need to keep up with the development of vaccine technology to improve mRNA and optimize its supply chain.
We can see that the project of mRNA technology today will gradually expand to a wider application in the future. Besides of the production of COVID-19 vaccines, it is also used for traditional vaccines (of influenza, rabies, herpes zoster, etc.), and will be widely applied to the treatment of tumor and autoimmune system. The vaccine companies in China are inspired to catch up by the global development trend, the favorable policies, the advantages of mRNA technical route and the outstanding effect of mRNA in addressing the global pandemic of COVID-19. Recently, the approval obtained by multiple companies for the clinical trials of COVID-19 mRNA vaccines again draws the attention of the industry.
Principle of mRNA vaccine technology
mRNA vaccine introduces mRNA containing encoded antigenic protein into human body, which is directly translated to form the corresponding antigenic protein, thus inducing specific immune response for preventive immunity. As the third generation following inactivated vaccine, live attenuated vaccine, subunit vaccine and viral vector vaccine, mRNA vaccine is characterized by fast response to pathogenic mutations, simple production process, easy production on a large scale and high safety and effectiveness.
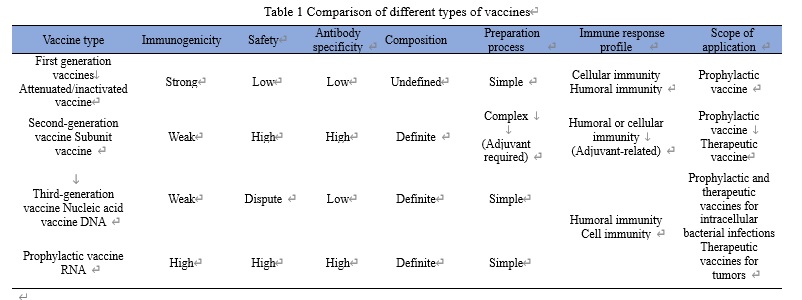
History of mRNA vaccine development
In 1989, an American company VICAL first used synthetic RNA as a novel drug for the treatment of tumors and other diseases. In 1990, a study showed that in vitro transcribed (IVT) mRNA could transmit genetic information and thus express proteins in body tissues by intramuscularly injecting naked mRNA into mice. Since the early 21st century, the development of mRNA synthesis, modification and delivery techniques has gradually brought mRNA technology back to the attention of scientists and biopharmaceutical companies. Since 2017, mRNA has demonstrated excellent anti-tumor abilities in clinical settings. In 2018, a biotech company Moderna achieved the largest IPO financing ever in biotech history based on the exploration of mRNA technology. In 2020, mRNA vaccine made a splash in the research and development of COVID-19 vaccines. In February 2021, 30 world-class scientists, including 15 Nobel Prize winners, highly praised mRNA vaccines. In addition, mRNA vaccine topped the list of “10 Breakthrough Technologies 2021” published by MIT Technology Review.
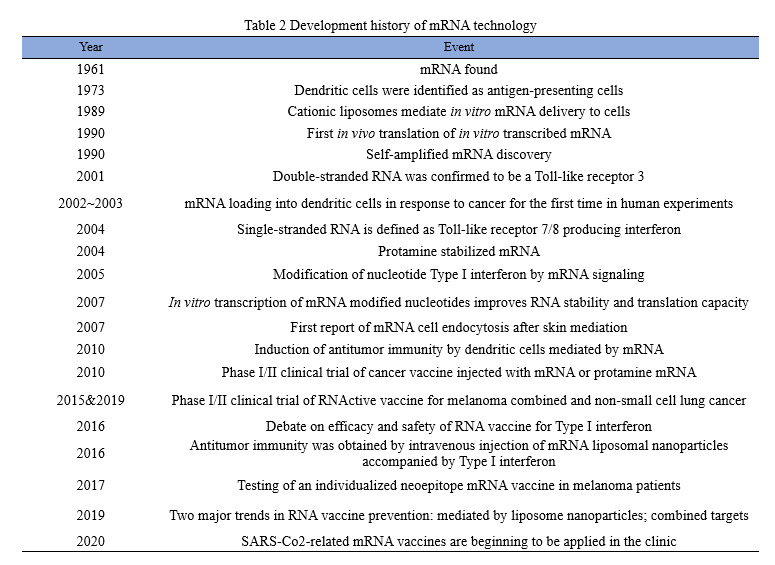
Trends in mRNA vaccine research, production and development
FiercePharma, a leading U.S. pharmaceutical website, has released a list of the world’s top 20 pharmaceutical companies based on revenue data for 2021. Among them, BioNTech and Moderna both made a name for themselves with their COVID-19 mRNA vaccines, with the former ranking 18th with $22.44 billion in revenue and the latter ranking 19th with $18.47 billion in revenue in 2021. In addition, some single products strongly boosted the sales of drug companies in 2021. Pfizer, for example, make a revenue of $81.29 billion in 2021, increasing by 95%, among which $36.781 billion was created by COVID-19 vaccines. It surpassed Humira of AbbVie and thus became the new “King of Medicine” around the world.
As Sean Marett, chief commercial officer of BioNTech, said: “All pharmaceutical companies are keeping a watchful eye on mRNA.” More than half of the world top 10 pharmaceutical companies, such as Pfizer, Roche, Johnson & Johnson, Sanofi, Merck, are cooperating with one another to work deeply in mRNA vaccines. As shown in the chart below, since the outbreak of COVID-19, listed pharmaceutical companies at home have also started a craze in collaborating with mRNA companies to develop vaccines.
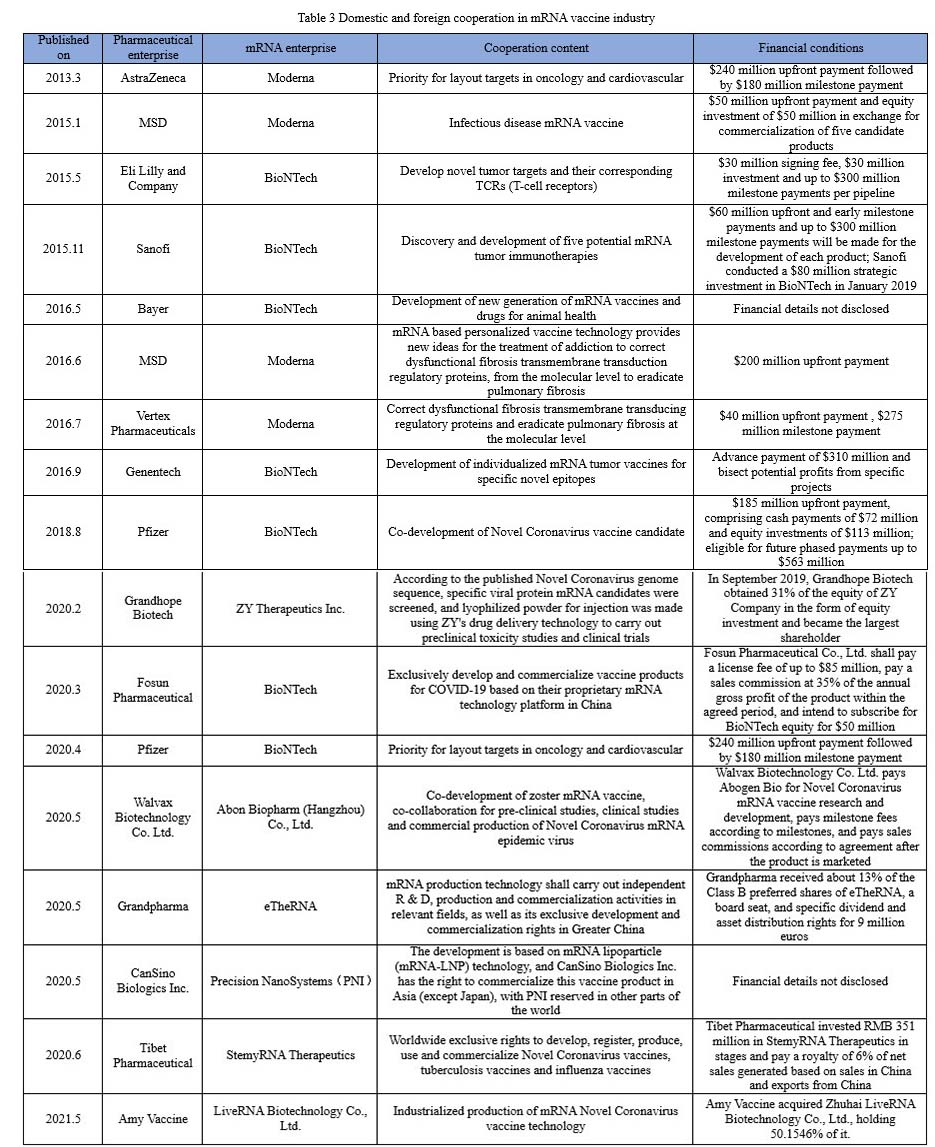
As of September 2021, Abbott Biologicals, Stemirna Therapeutics, Lifanda Biotechnology, CSPC, and CanSinoBio all had products of COVID-19 mRNA vaccines that were in clinical trials.
Production process of COVID-19 mRNA vaccines
The production process of mRNA vaccines differs from that of traditional vaccines or protein products based on cell culture in steps such as the production of mRNA bulk and LNP preparation. As the main component producing antigens in vaccines, mRNA molecules are generally produced through in vitro transcription (IVT) system. An appropriate purification process is then adopted to develop the vaccine products by lipid nanoparticle technology.
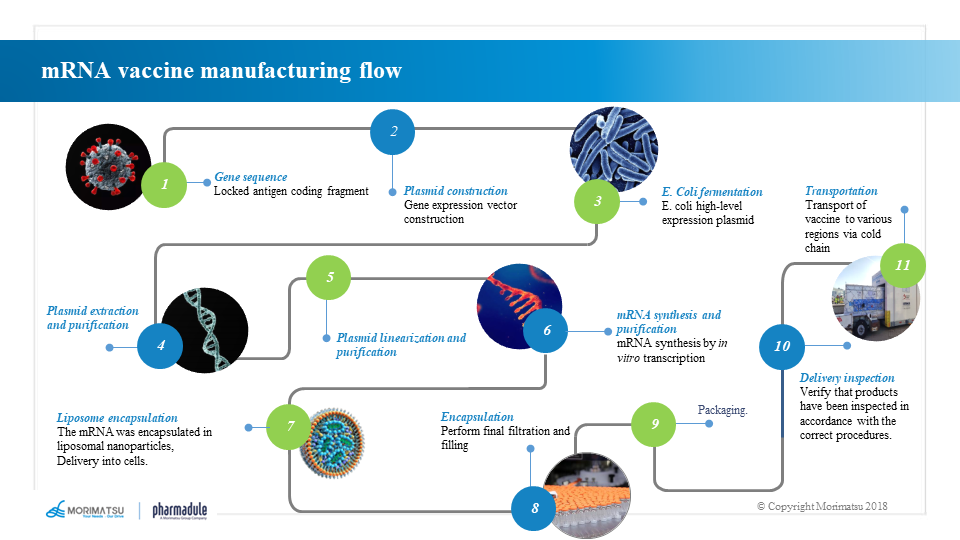
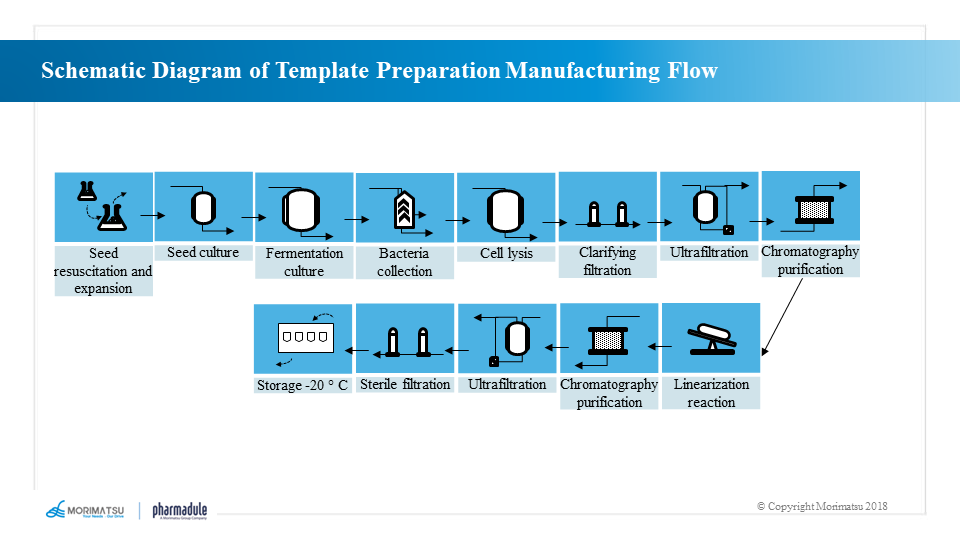
Template preparation and production processes
Sequential DNA templates are generally produced through steps including seed recovery → seed proliferation → fermentation → bacterial collection → bacterial lysis → clarification and filtration → plasmid purification → plasmid linearization → template purification.
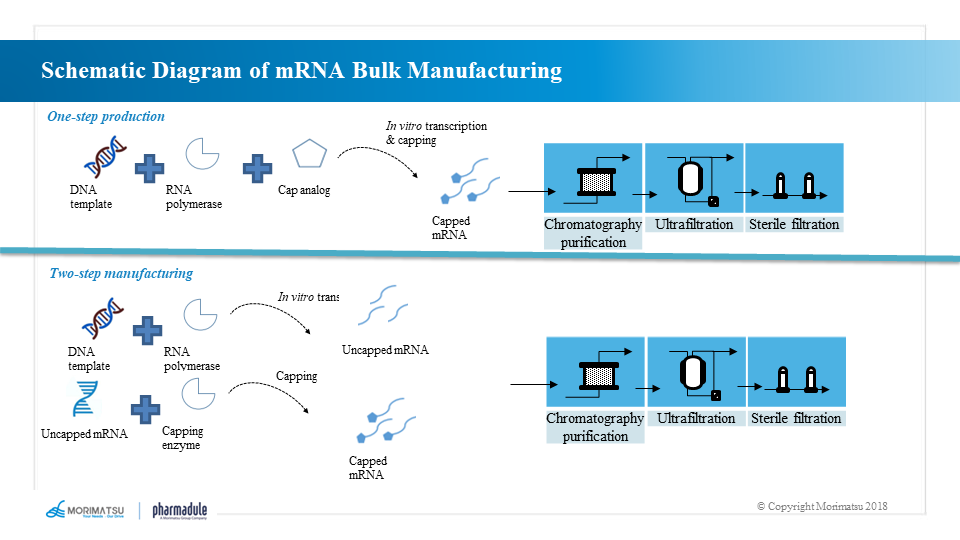
Production process of mRNA bulk
mRNA molecules are generated by the method of in vitro transcription (IVT), which takes DNA as the template and uses RNA polymerase including T7, T3 or Sp6 bacteriophage polymerase to prepare an open reading frame with protein encoding function, which includes a 5' “cap” structure, a 3' poly A “tail” and an untranslated region.
The mRNA bulk is generally produced by one-step or two-step synthetic reaction. The one-step method involves adding the synthesized 5’ cap structure to the starting sequence of specific transcription to directly transcribe the mRNA with the natural cap structure and then purify it to obtain the mRNA bulk. The two-step method first synthesizes the sequence without 5’ cap structure and purifies it, and then performs a capping reaction and purifies it again to produce the mRNA bulk.
Production process of mRNA bulk:
- One-step method: in vitro transcriptional reaction → chromatographic purification → ultrafiltration → clarification and filtration → sterilization and filtration
- Two-step method: in vitro transcriptional reaction → chromatographic purification → ultrafiltration → capping reaction → chromatographic purification → ultrafiltration → clarification and filtration → sterilization and filtration
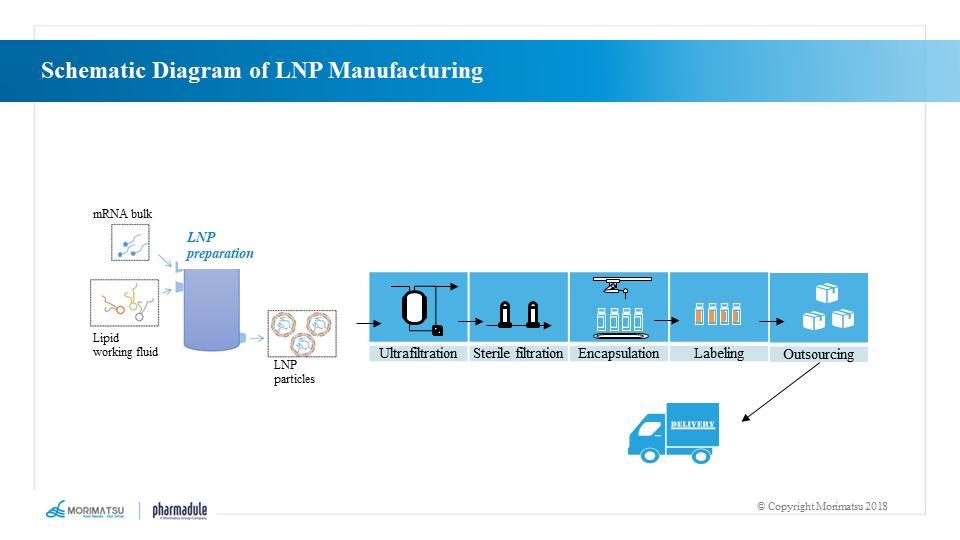
LNP preparation and filling processes
The effective in vivo delivery of mRNA is critical to the effectiveness of mRNA vaccines. Exogenous mRNA must penetrate the lipid membrane to enter the cytoplasm and be successfully translated into functional proteins.
LNPs are lipid-based delivery systems that consist of cationic lipids, co-lipids, cholesterol and polyethylene glycol (PEG) to form stable particles that carry mRNA in the core and protect it from degradation. In addition, the lipotropism of LNP allows the particles to fuse with the cytomembrane of the subject and thus deliver mRNA into the subject’s cells.
The production process of finished mRNA vaccine is as follows:
- LNP encapsulation → ultrafiltration → drug product production → sterilization and filtration → filling → packaging of finished products
Precautions of process research and development for engineering realization of mRNA vaccines
- The concentration of products by fermentation culture. In order to improve the yield of the template, it is necessary to select highly expressive bacteria, while considering highly expressive fermentation conditions and equipment.
- Selection of mRNA synthesis method. Simple, accessible and stable synthesis methods are more conducive to the engineering production of mRNA vaccines.
- Optimization and improvement of purification route. The major purification techniques adopted by the production of mRNA vaccines are chromatography and ultrafiltration. A plan with fewer purification steps may be selected for the template preparation to enhance the yield.
- Production capacity matching of each stage in mRNA vaccine production platform. The production of mRNA vaccine consists of four main stages, i.e. template production, mRNA bulk production, drug product production, and filling & packaging. Relatively speaking, the capacity of the filling system is usually the capacity bottleneck of the system. As to the design of production platform, it is necessary to link the upstream and downstream processes and comprehensively consider the capacity design of each stage to fully satisfy the requirements for capacity and reduce unnecessary redundancy.
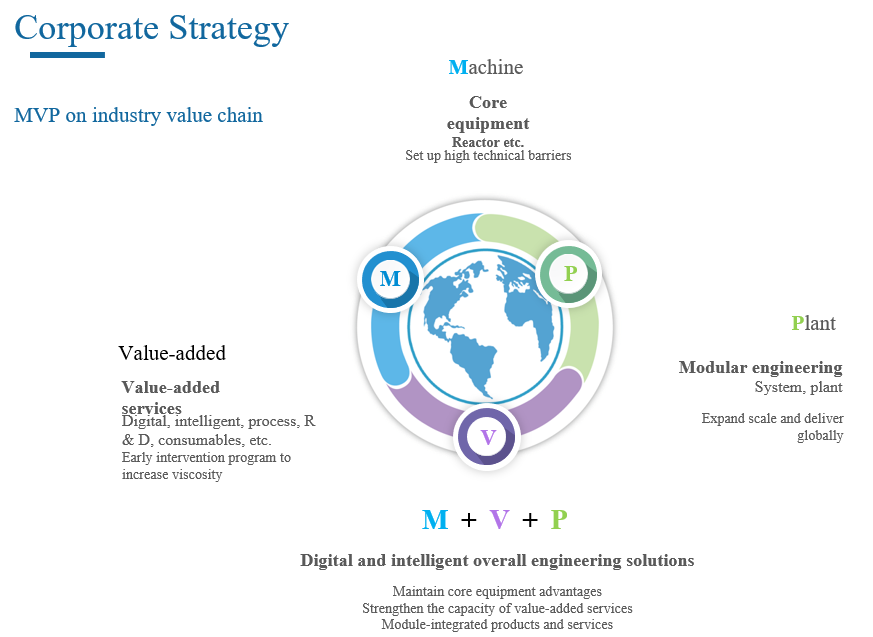
Opportunities for Morimatsu in the wave of mRNA projects
Based on its strategic MVP model, Morimatsu can provide digital and intelligent overall engineering solutions integrating products with services for mRNA projects. Where, the products include plants, process systems and core equipment (such as fermenter) delivered in modular form, while the value-added services include an all-round design service covering concept design, basic design, detailed design and modular design, and a post-delivery digital operation and maintenance service.
The digital and intelligent overall engineering solutions of Morimatsu depending on the production platform of mRNA vaccines help customers achieve lean production by linking upstream and downstream processes, improving production efficiency and increasing the production efficiency of the engineering platform. We also provide a full life-cycle process ranging from digital design, construction to operation and maintenance to create a future model plant for biopharmaceutical production in China and even the world for customers.
One of value-added services - design
With the strong technical support from Pharmadule in Sweden in process design and modular plant design, Morimatsu has the most cutting-edge industry concepts and advanced engineering technologies. It can provide customers with “tailored” design plans for production systems according to the process information and capacity requirements of mRNA production provided by customers. The lean production scheduling will help customers optimize process design and layout and thus realize the automation and digitalization of plants. This not only helps domestic customers to keep in line with the requirements of the National Medical Products Administration (NMPA), but also enriches their knowledge and experience in interpreting and satisfying the Good Manufacturing Practice (GMP) of WHO, the European Medicines Agency (EMA) and the Food and Drug Administration (FDA).
Core equipment
Morimatsu can provide diversified products for mRNA production, including fermenter systems, lysis systems, purification systems, ultrafiltration systems, drug product dispensing systems, buffer preparation systems, on-line dispensing systems, inactivation and CIP workstation systems, isolators, disposable magnetic mixing systems, etc.

Modular plant
What can we see from the trend? The urgent need for agile, flexible and fast engineering delivery. The first mRNA COVID-19 vaccine modular chemical plant in China delivered by Morimatsu in 2021 was successfully completed in 7.5 months. With a building area of more than 5,000 m2, this plant involves the entire production process ranging from the plasmid production, bulk preparation, drug product production to filling.

Due to the modular construction, more than 80% of the work can be finished off-site regardless of the external environment. The advantages in Chinese stable supply chain and Chinese engineers and workers can be taken to the utmost to better ensure the faster project delivery with lower risk and the predictable project implementation with greater flexibility.

Facing the continuous changes of COVID-19 around the world, all the countries take vaccines as strategic national supplies and rise to build local biopharmaceutical plants to create a localized vaccine supply chain. The implementation mode of the project has been noticed by global biopharmaceutical industry and is cited by numerous companies.
As mRNA technology is widely applied to the research and development of COVID-19 vaccines in the circumstance of recurring COVID-19 pandemic, it is justified to define it as the key to the future. Opportunities follow upon challenges. Under the rapidly changing situation, Morimatsu closely follows the trend and firmly seizes the opportunities to constantly contribute to the fight against the pandemic and to the improvement of human health with its expertise in the field.
About Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd.
Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd (Stock Code: 2155.HK) is a wholly-owned subsidiary of Morimatsu International Holdings Company Limited. Morimatsu, founded in Japan and taken root in China, has developed into a multi-national company that embraces globalization, masters core technology, and gains rich experience from project implementation in diverse fields including core equipment, process systems, and digital and intelligent engineering solutions. The company has built advanced manufacturing base in China, established affiliates in Sweden, the United States, India, and Italy, built an efficient professional globalization team, and delivered a variety of products and services to more than 40 countries and regions.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of “predicted”, “believed”, “forecast”, “planned” and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our company management’s current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guaranties of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.















