In August, 2017, FDA approved Kymriah as the world's first CAR-T product, which is used to treat patients with relapsed or refractory acute lymphoblastic leukemia aged 3-25.This cell therapy product has a short treatment process, precise targeting, and long-lasting lethality and activity. It is another blockbuster drug type after small molecules and macromolecules, and has great prospects.
Technological process of cell therapy production
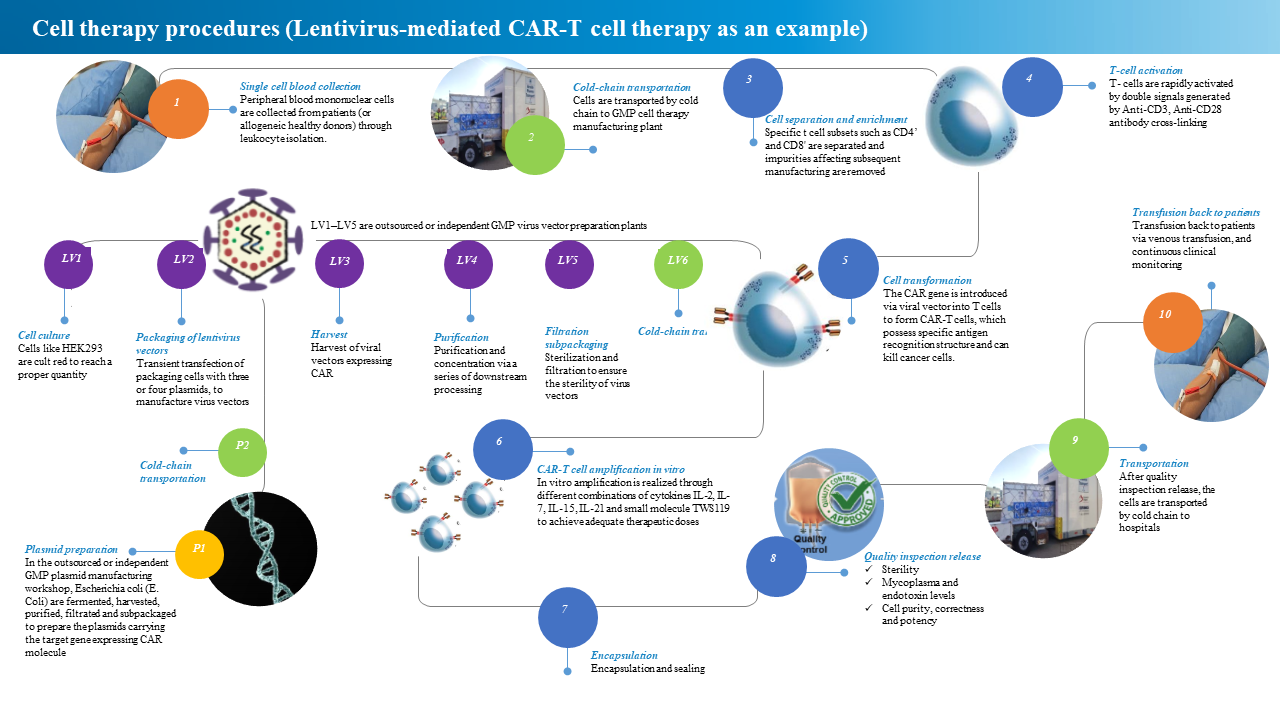
Cell therapy is applied to human autologous or allogeneic derived cells after in vitro manipulation (or implantation) into the human body for disease treatment.Taking chimeric antigen receptor T cell (hereinafter referred to as CAR-T) therapy as an example, its production mainly includes three major links: plasmid production, viral vector production (delivery system) and cell preparation production.
From the perspective of cell preparation, the production of CAR-T begins with the collection of peripheral blood mononuclear cells from patients (or allogeneic donors) through leukocyte isolation. This step is usually carried out in the hospital.These cells are then cold-chained to the cell therapy production facility, where specific cell subsets (such as CD4+, CD8+ T) are separated and impurities (such as anticoagulants, red blood cells, etc.) that affect subsequent cell production are removed.Then T cells are activated by antibody coupling (such as CD3, CD28) to generate specific signals.Next is the expression of CAR on T cells. This is the core step of CAR-T production, which requires the use of a vector to introduce the CAR gene into T cells.Today, there are different ways including viral vector transduction, such as lentiviral vector and non-viral vector transfection, such as transposon transfection, electroporation technology and so on.After obtaining stable CAR-T cells, large-scale in vitro expansion is also required to obtain the dose required for treatment. Finally, after being packaged, frozen and released through a series of quality inspections, it is transported to the hospital by cold chain and then infused back to the patient.
The lentiviral vector required for cell transformation has its own preparation process, which includes culturing HEK293 cells to a certain density after resuscitation, and then transiently transfecting the packaged virus with three or four plasmids. After the packaged virus is harvested, it is prepared by a series of purification and sterilization filtration.The plasmids, which are essential elements of viral vectors, need to be prepared from Escherichia coli fermentation, harvesting, purification, and sterile filtration.
Factors to consider in the construction of cell therapy industrialization
At the beginning of the industrialization of cell therapy, many factors need to be considered:
1. Cell therapy is usually customized production for individual patients, and changes in time will affect the disease progression of patients and the treatment measures of doctors, posing challenges to the production time and maintenance of drug efficacy of CAR-T therapy.
2. Plasmid, virus vector and cell preparation are indispensable in the preparation of CAR-T products. Enterprises need to consider whether to outsource the production of plasmids and viral vectors from the perspective of industrialization strategy.If the enterprise produces by itself, the plasmid, virus vector and cell preparation require separate production areas, which puts forward higher requirements for the production, storage, internal transport and external transport of drugs.
3. At the beginning of the construction of the production plant, it is necessary to consider minimizing the pollution and risk, which means that many factors such as the stability of environmental conditions, the risk assessment of pollution and the availability of environmental data during the production process will affect the final efficacy of the product.For example, in multiple links where living cells need to be processed, the traditional practice is to use a Class B clean room to handle the product under the protection of an open Biological Safety Cabinet (BSC).In the future, more closed protection will be used to reduce the risk of pollution and cross infection. The isolator will be placed in the background C/D clean room, the product is under the A-level protection inside the isolator compartment, and the operator does not directly touch the product but operates through the glove port.Therefore, different protection strategies have an impact on the process layout of the workshop and the future operating procedures.
4. Due to the particularity of the product, it is necessary to be more strict in quality research, safety monitoring and regulatory supervision. Quality monitoring should run through the whole life cycle of drugs, and complete quality inspection facilities are required.
5. It is necessary to consider the planning of the product in the next 5 to 10 years or even longer. At the beginning of production, the annual production capacity covers a small number of patients, and once the therapy is successful, the production capacity increases rapidly to meet the demand.Therefore, it is particularly important that the reasonable construction of production facilities not only meet the expected product quality and plant capacity, but also have the scalability and variability of future operations.

Morimatsu isolator with robotic workstation
Solutions and technical advantages provided by Morimatsu in cell therapy industrialization projects
Morimatsu, who has been deeply involved in the biological industry for many years, now has the most cutting-edge industry concept, advanced engineering technology and rich GMP experience based on its profound interpretation of the cell therapy industry.At the beginning of plant design, we can base ourselves on the present and have insight into the future, and continue to provide customers with preliminary plant planning consultation and process design layout services with quality derived from design.
With its solid foundation in the professional field, in the construction of cell therapy industrialization, Morimatsu can customize flexible overall engineering solutions according to the different needs of customers, and provide engineering design, core equipment, engineering systems and modular plants from plasmid production, virus vector production to cell preparation production.Among them, the modular leading technology, taking advantage of the advantages of remote construction, can integrate process equipment, clean workshop, utilities and automatic control while building the plant, which greatly saves the customer's on-site installation and commissioning time and accelerates the overall construction cycle of the project.At the same time, modularity has excellent scalability and flexibility, which can help pharmaceutical enterprises shape their core competitiveness, seize the development track, and make more contributions to the rapid production and popularization of advanced biological drugs.

Modular cell therapy plant
About Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd.
Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd (Stock Code: 2155.HK) is a wholly-owned subsidiary of Morimatsu International Holdings Company Limited.Morimatsu, founded in Japan and taken root in China, has developed into a multi-national company that embraces globalization, masters core technology, and gains rich experience from project implementation in diverse fields including core equipment, process systems, and engineering solutions.The company has built advanced manufacturing base in China, established affiliates in Sweden, the United States, India, and Italy, built an efficient professional globalization team, and delivered a variety of products and services to more than 40 countries and regions.
Forward-Looking Statements
The information in this press release may include some forward-looking statements.Such statements are essentially susceptible to considerable risks and uncertainties.The use of “predicted”, “believed”, “forecast”, “planned” and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones.Our company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our company management’s current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements.Such statements are not guaranties of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our company and unpredictable.Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.







